
The ion name is sodium cation or sodium ion
/molecular-structure-of-glucose-85758435-58b8801a5f9b58af5c28e946.jpg)
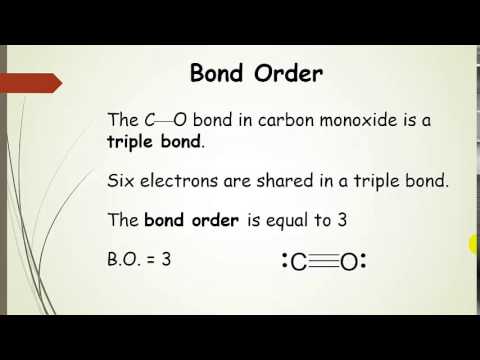
Question: Calculate the net ionic charge of Na + It also has information about chemical quantities, unit conversion, molarity calculations and stoichiometry. With WolframAlpha, you can explore data about chemical compounds, the reactions they undergo, solubility and chemical graph theory. Criss-cross Method: Write the symbol, charge of the cation first and anion second. Chemistry is the study of matter, from individual atoms and ions to large biomolecules. The combination of the cycles and double or triple bonds in your molecule. Write the final formula and all charges and subscripts that are 1. Constitutional isomers are molecules that have the same molecular formula but. These consist of carbon-hydrogen bonds and also carbon to carbon single bonds where these carbon atoms join forming a ring or in the shape of a cyclic structure. Write those multipliers as subscripts for each ion. They have the general formula C n H 2n,Where n is said to be the number of carbon atoms present in the organic compound. Use a multiplier to make the number of cations and anions are equal. The ionic formula can be written in 2 ways, one is general method and the other is a criss-cross method. At first, write the symbol and charge of the cation and anion. The net ionic charge is nothing but the number of cations or anions a chemical compound is having. In an ionic compound, the number of cations and anions are equal. The positively charged ions are called cations and negatively charged ions are called anions. We need two pieces of information to do this calculation. The formed compound is neutral as it has positively charged ions and negatively charged ions. The chemical formula for octane is: CH3 (CH2)6 CH3 Calculate the molar mass of octane Round. Covalent chemical bonds involve the sharing of a pair of valence electrons by two atoms, in contrast to the transfer of electrons in ionic bonds. An ionic compound is defined as a chemical compound that is made up of ions that are held together by the electrostatic force known as ionic bonding.


 0 kommentar(er)
0 kommentar(er)
